Introduction
Good day everyone!
In this tutorial, we will review IJMB Chemistry 2016 past questions focusing on mole calculations, molecular ions, and fundamental chemical constants. These are common areas tested in IJMB exams and are crucial for students preparing for university direct entry programs.
Let’s get started with the questions and answers.
Question 1: Mole Calculation for Copper Formation
Question:
Calculate the number of moles of copper formed when 20.50 g of copper(II) oxide completely reacts with hydrogen gas.
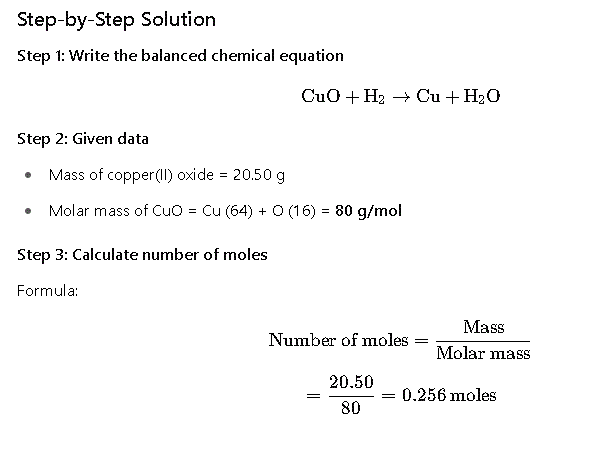
Final Answer
The number of moles of copper formed is 0.256 moles.
Question 2: Define Molecular Ion
Answer:
Molecular ions, also called radicals or polyatomic ions, are groups of atoms bonded together that carry an overall charge.
Examples:
- NH₄⁺ (Ammonium ion)
- CO₃²⁻ (Carbonate ion)
- SO₄²⁻ (Sulfate ion)
These ions behave as single units in chemical reactions.
Question 3: Number of Atoms in Three Moles of Cobalt
Question:
How many atoms are there in three moles of cobalt?

Watch more educational videos and past questions: https://youtube.com/@allcbts
Conclusion
Mastering mole concepts, molecular ions, and Avogadro’s constant is vital for scoring high in IJMB Chemistry exams. By understanding the principles behind these questions, students can tackle similar problems confidently during exams.
Related Videos:
- Understanding Waves and Properties in Physics
- Understanding DNA and Genetics in Biology
- Physics WAEC Past Questions
- NCEE 2025 General Paper Questions
- Chemistry Practical Past Questions
Keywords
- IJMB chemistry past questions and answers
- mole calculation copper(II) oxide IJMB 2016
- Avogadro’s constant and number of atoms
- definition and examples of molecular ions
- IJMB direct entry chemistry likely questions
- polyatomic ions explained for IJMB students
- how to solve mole concept problems in chemistry
- past chemistry questions for IJMB exams
- copper(II) oxide reaction with hydrogen explained