Introduction
Welcome JEE aspirants!
In today’s chemistry tutorial, we’ll walk through a solved JEE-level chemistry question involving combustion analysis to calculate the percentage composition of hydrogen and oxygen in an organic compound.
This concept is crucial for excelling in:
- JEE Main Chemistry
- JEE Advanced Chemistry
- CBSE Class 11 & 12 Organic Chemistry
- Engineering entrance exams across India
Let’s proceed with a methodical, exam-focused approach to mastering this core topic.
You can watch the full class in the video below:
Question Overview
Problem:
On combustion, 0.98 g of an organic compound (containing carbon, hydrogen, and oxygen) yields 0.27 g of water (H₂O) and 0.7 g of carbon dioxide (CO₂).
You are asked to calculate the percentage composition of hydrogen and oxygen in the compound.
Step 1: Data Extraction
- Mass of compound = 0.98 g
- Mass of water (H₂O) = 0.27 g
- Mass of carbon dioxide (CO₂) = 0.7 g
Step 2: Determine Mass of Hydrogen
Since hydrogen comes from water, we calculate its mass based on the molecular ratio:
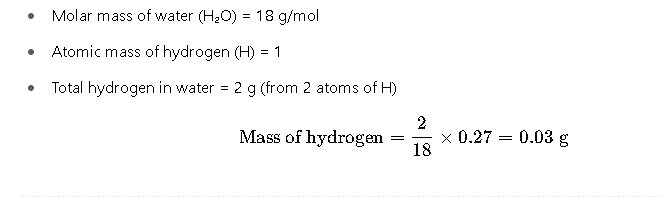
Step 3: Determine Mass of Carbon
Carbon is derived from carbon dioxide (CO₂):
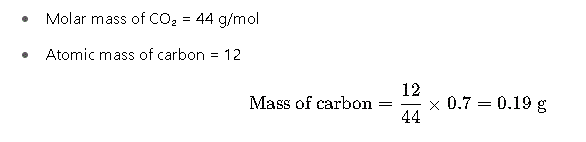
Step 4: Calculate Mass of Oxygen
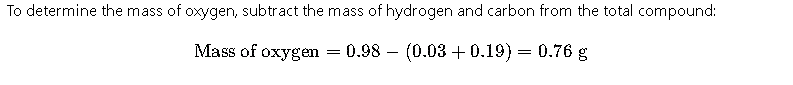
Step 5: Calculate Percentage Composition
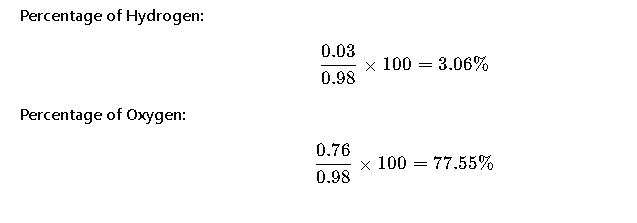
Final Answer
- Hydrogen: 3.06%
- Oxygen: 77.55%
Watch more educational videos and past questions: https://youtube.com/@allcbts
Conclusion
Understanding how to calculate the percentage composition of elements through combustion analysis is a vital skill for success in JEE Main and Advanced Chemistry.
In this tutorial, we:
- Identified the role of water and carbon dioxide in combustion
- Used molar mass ratios to calculate elemental masses
- Applied stoichiometric principles to find percentage composition
- Keep practicing similar questions and focus on concepts like empirical formula, mole calculations, and organic compound identification — all of which are frequently tested in JEE exams.
Related Videos:
- Understanding Waves and Properties in Physics
- Physics 100-Level Past Questions
- GAOKAO Top Mathematics Questions
- Quantitative Reasoning Questions for Basic School
- NCEE Verbal Reasoning for Common Entrance Exam
SEO Keywords for JEE Chemistry
- JEE chemistry percentage composition questions
- combustion analysis JEE main
- hydrogen and oxygen composition in organic compounds
- JEE Advanced chemistry solved examples
- stoichiometry questions for JEE
- organic compound analysis JEE
- JEE chemistry mass-mass relationship

 No products in the cart.
No products in the cart.